Answer: Boiling point
Explanation:
Boiling point is the temperature where the vapor pressure of a liquid equals the pressure of the gas present above it.
The normal boiling point of a liquid is the temperature at which its vapor pressure is equal to one atmosphere.
Boiling point depends on the intermolecular forces present between the molecules. More are the intermolecular forces, lesser will be the vapor pressure and thus more heat will be supplied to make vapor pressure equal to the atmospheric pressure. Thus higher will be the boiling point.
Normal boiling point for water is
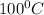 .
.