Answer : The correct option is, (c) inversely proportional to its volume
Explanation :
Boyle's Law : It is defined as the pressure of the gas is inversely proportional to the volume of the gas at constant temperature and number of moles.
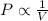
Gay-Lussac's Law : It is defined as the pressure is directly proportional to the temperature of the gas at constant volume and number of moles.
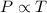
Charles' Law : It is defined as the volume is directly proportional to the temperature of the gas at constant pressure and number of moles.
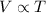
Hence, for a given ideal gas if it temperature is constant the pressure of the gas is inversely proportional to its volume.