Answer:
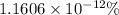 is the percentage of the volume of the atom is occupied by the nucleus.
is the percentage of the volume of the atom is occupied by the nucleus.
Step-by-step explanation:
Radius of the nucleus of the hydrogen atom,
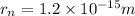
Radius of the hydrogen atom =
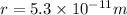
Volume of hydrogen atom ,V=
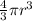
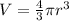
Volume of nucleus of hydrogen atom ,
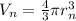
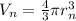
Percentage of the volume of the atom is occupied by the nucleus:
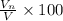
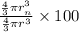
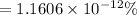
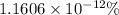 is the percentage of the volume of the atom is occupied by the nucleus.
is the percentage of the volume of the atom is occupied by the nucleus.