Answer:
No. of moles, n = 25.022 moles
Given:
Volume of gas in tank, V = 29.1 l
Temperate of gas, T =
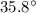 = 273 + 35.8 = 308.8 K
= 273 + 35.8 = 308.8 K
Pressure of gas, P = 21.8 atm
Solution:
Making use of the ideal gas equation which given as:
PV = nRT
where
R = Rydberg's constant = 0.0821 L-atm/mol-K
Re-arranging the above formula for 'n' and putting the values in the above formula:
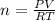
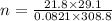
n = 25.022