Answer:
The disintegrations is
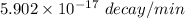 .
.
Step-by-step explanation:
Given that,
Weight of sample = 200 g
Decay constant
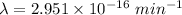
We need to calculate the disintegrations
Using formula of disintegrations
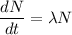
Where,
 = decay constant
= decay constant
N = number of atoms present at time t
Put the value into the formula
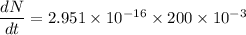
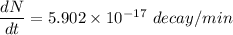
Hence, The disintegrations is
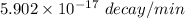 .
.