Answer : The volume required is, 25 mL
Explanation :
First we have to determine the mass of potassium present in 100 mL or 0.1 L of each standards.
For 25 mg/L :
As, 1 L volume has mass of potassium = 25 mg
So, 0.1 L volume has mass of potassium =
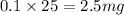
For 50 mg/L :
As, 1 L volume has mass of potassium = 50 mg
So, 0.1 L volume has mass of potassium =
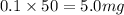
For 75 mg/L :
As, 1 L volume has mass of potassium = 75 mg
So, 0.1 L volume has mass of potassium =
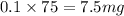
For 100 mg/L :
As, 1 L volume has mass of potassium = 100 mg
So, 0.1 L volume has mass of potassium =
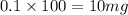
Now we have to determine the total mass of potassium.
Total mass of potassium = 2.5 + 5.0 + 7.5 + 10 = 25 mg
Now we have to determine the required volume of the 1000 mg/L Potassium standard solution.
As, 1000 mg mass of potassium present in 1 L volume
So, 25 mg mass of potassium present in
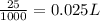 volume
volume
Volume required = 0.025 L = 25 mL (conversion used : 1 L = 1000 mL)
Therefore, the volume required is, 25 mL