Step-by-step explanation:
It is given that,
An electron is located on a pinpoint having a diameter of 3.52 µm,
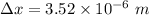
We need to find the minimum uncertainty in the speed of the electron. It can be calculated using Heisenberg uncertainty principal as :
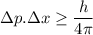
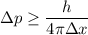
Since, p = m v
So,
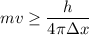
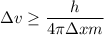
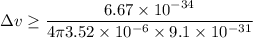
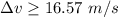
So, the minimum uncertainty in the speed of the electron is greater than 16.57 m/s. Hence, this is the required solution.