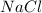Answer: B. the atoms have an unequal sharing of the electrons
Step-by-step explanation:
Electronegativity is defined as the property of an element to attract a shared pair of electron towards itself.
A polar moleucle is defined as a molecule which is formed when there is a difference of electronegativities between the atoms which leads to unequal sharing of electrons. example:
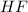
Non-polar covalent molecule is defined as a molecule which is formed when there is no difference of electronegativities between the atoms which leads to equal sharing of electrons.Example:

Ionic bond is formed when there is complete transfer of electron from a highly electropositive metal to a highly electronegative non metal resulting into a neutral compound. Example;