Answer:
93 moles
Step-by-step explanation:
The breakdown of 1 mole of glucose is shown by the reaction shown below:
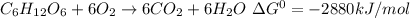
It means that 1 mole of breakdown of glucose produces 2880 kJ of energy.
The reaction of the phosphorylation of ADP to ATP is shown below as:
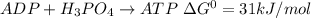
It means that 1 mole of ADP needs 31 kJ of energy to convert into 1 mole of ATP
Which also means that:
31 kJ of energy is required to form 1 mole of ATP from ADP
1 kJ of energy is required to form
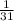 mole of ATP from ADP
mole of ATP from ADP
2880 kJ of energy is required to form
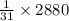 mole of ATP from ADP
mole of ATP from ADP
Thus moles of ATP form ≅ 93 moles