Answer:
For a: The energy corresponding to 400 nm is 2.06 eV and energy corresponding to 600 nm is 3.10 eV.
For b: The binding energy of the metal is 3.10 eV.
Step-by-step explanation:
The equation used to calculate the energy for given wavelength, we use the equation given by Planck, which is:
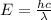
To convert into MeV, we use the final equation:
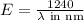 .......(1)
.......(1)
- Energy associated with purple light having wavelength 400 nm
Putting values in equation 1, we get:
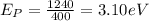
- Energy associated with orange light having wavelength 600 nm
Putting values in equation 1, we get:
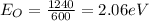
When two lights are made to fall on metal, photons on each color collides with the metal electrons individually. The energy associated with them do not get add up.
As, the energy of purple light is higher. So, the photons of this color will manage to bring the electrons of metal to come to the surface and hence, this color will give use the value of binding energy.
Energy associated to purple color = Binding energy
Thus, the binding energy of the metal is 3.10 eV.