Answer:
The correct answer is option e.
Step-by-step explanation:
The formula used for root mean square speed is:
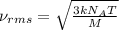
where,
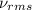 = root mean square speed
= root mean square speed
k = Boltzmann’s constant =
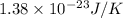
T = temperature
M = Molar mass
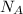 = Avogadro’s number =
= Avogadro’s number =
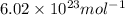
Root mean square speed of carbon dioxide molecule:
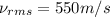
Temperature of the mixture = T =?
Molar mass of carbon dioxide = 44 g/mol = 0.044 kg/mol
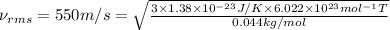
T = 533.87 K
Root mean square speed of nitrogen molecule:
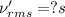
Molar mass of nitrogen = 28 g/mol = 0.028 kg/mol
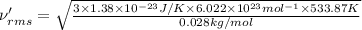
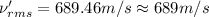
689 m/s is the root-mean-square speed of the nitrogen molecules in the sample.