Step-by-step explanation:
Molecular mass of
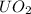 is the sum of molecular mass of U and
is the sum of molecular mass of U and
 molecule.
molecule.
Molar mass of
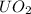 = (238 + 32) g/mol
= (238 + 32) g/mol
= 270 g/mol
Hence, in 1 kg there are 1000 grams. So, total number of atoms given molecules present in 1 kg will be calculated as follows.
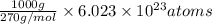
=
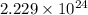
Hence, number of
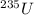 =
=
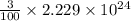
=
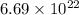
Now, let x seconds is required for burning 100 W lamp bulb. As 200 MeV is released per reaction.
100 x =
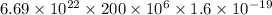
x =
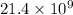 sec
sec
Converting this value of x into years as follows.
x =
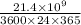
x =
 years
years
Thus, we can conclude that fissioning of the uranium in given situation requires
 years to keep a 100 W lamp burning.
years to keep a 100 W lamp burning.