Answer:
There are 0.2972 moles of chlorine in
 atoms of chlorine.
atoms of chlorine.
Step-by-step explanation:
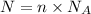
Where:
N = Number of particles / atoms/ molecules
n = Number of moles
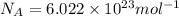 = Avogadro's number
= Avogadro's number
We have:
N =
 atoms of chlorine
atoms of chlorine
n =?
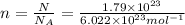
n = 0.2972 moles
There are 0.2972 moles of chlorine in
 atoms of chlorine.
atoms of chlorine.