Answer:
Difference in atomic mass of Al and Pb makes their densities different
Step-by-step explanation:
We know that
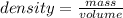
Here volume of both Al and Pb are
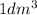
Again they have similar number of atoms in
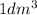 volume
volume
But atomic mass of Al is 27 amu and atomic mass of Pb is 207 amu.
Mass of an element in
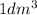 volume would be summation of atomic masses of all atoms present.
volume would be summation of atomic masses of all atoms present.
As difference in atomic masses between Al and Pb is quite high therefore it is expected that their densities would be very different.