Step-by-step explanation:
A compound is a substance that contains more than two different atoms in a fixed ratio by mass. Elements of a compound are chemically combined together.
A homogeneous mixture is defined as a mixture in which solute particles are uniformly distributed into the solvent.
Whereas a heterogeneous mixture is defined as a mixture in which solute particles are non-uniformly distributed in a solvent.
In both homogeneous and heterogeneous mixture there is no chemical combination between solute and solvent particles.
Therefore, each given substance is classified as follows.
(a) Orange juice : As it contains pulp of orange along with the liquid which are not present in uniform composition. Hence, orange juice is a heterogeneous mixture.
(b) vegetable soup : This soup also contains vegetable pieces and spices which are non-uniformly distributed. Hence, it is also a heterogeneous mixture.
(c) cement : It contains several different compound present in definite composition. Hence, cement is a homogeneous mixture.
(d) calcium sulfate : Its chemical formula is
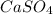 and in this compound both calcium and sulfate atoms are chemically combined together. Therefore, calcium sulfate is a compound.
and in this compound both calcium and sulfate atoms are chemically combined together. Therefore, calcium sulfate is a compound.
(e) Tea : It is a homogeneous mixture as there is uniform composition of tea water in milk.