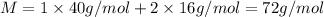Answer:
a) The molecular mass of each compound dinitrogen pentaoxide is 108 g/mol.
b) The molecular mass of each compound lead(ll) nitrat is 331 g/mol.
c) The molecular mass of each compound calcium peroxide is 72 g/mol.
Step-by-step explanation:
a) Molecular mass of dinitrogen pentaoxide that
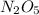 be M.
be M.
Mass of nitrogen atom = 14 g/mol
Mass of oxygen atom = 16 g/mol
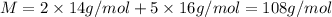
b) Molecular mass of lead(ll) nitrate that
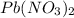 be M.
be M.
Mass of lead = 207 g/mol
Mass of nitrogen atom = 14 g/mol
Mass of oxygen atom = 16 g/mol
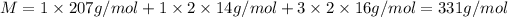
c)Molecular mass of calcium peroxide that
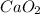 be M.
be M.
Mass of calcium atom = 40 g/mol
Mass of oxygen atom = 16 g/mol