Answer: The salt produced will be
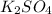
Step-by-step explanation:
During a neutralization reaction, an acid reacts with a base for producing the correspondent salt, and water.
The strong acids release all the protons avalaible when are dissolved, such as sulfuric acid. As you can see, sulfuric acid have 2 protons ready for being released (
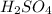 ); and those places have to be occcupied for other ions equivalents to the H+: K+ from KOH in this case.
); and those places have to be occcupied for other ions equivalents to the H+: K+ from KOH in this case.
Therefore the answer will be
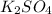 .
.