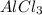Answer:
For a: The chemical name and chemical formula formed is sodium nitride and
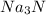
For b: The chemical name and chemical formula formed is strontium oxide and
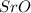
For c: The chemical name and chemical formula formed is aluminium chloride and
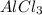
Step-by-step explanation:
For formation of a neutral ionic compound, the charges on cation and anion must be balanced. The cation is formed by loss of electrons by metals and anions are formed by gain of electrons by non metals.
The nomenclature of ionic compounds is given by:
1. Positive is written first.
2. The negative ion is written next and a suffix is added at the end of the negative ion. The suffix written is '-ide'.
- For a: Sodium and nitrogen:
Sodium is the 11th element of periodic table having electronic configuration of
![[Ne]3s^1](https://img.qammunity.org/2020/formulas/chemistry/high-school/wfm0xsh6s3dkxk5blgxjomh7cl0mn0ln0e.png)
To form
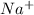 ion, this element will loose 1 electron.
ion, this element will loose 1 electron.
Nitrogen is the 7th element of periodic table having electronic configuration of
![[He]2s^22p^3](https://img.qammunity.org/2020/formulas/chemistry/college/3au485vm0mx09i1d8rsz0c5t5l8ylclldu.png) .
.
To form
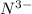 ion, this element will gain 3 electrons.
ion, this element will gain 3 electrons.
By criss-cross method, the oxidation state of the ions gets exchanged and they form the subscripts of the other ions. This results in the formation of a neutral compound.
So, the chemical formula for sodium nitride is
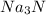
- For b: Oxygen and strontium
Strontium is the 38th element of periodic table having electronic configuration of
![[Kr]5s^2](https://img.qammunity.org/2020/formulas/chemistry/college/702fsvsyrkanv1tcgi6ipda6f0jeba8w6r.png) .
.
To form
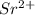 ion, this element will loose 2 electrons.
ion, this element will loose 2 electrons.
Oxygen is the 8th element of periodic table having electronic configuration of
![[He]2s^22p^4](https://img.qammunity.org/2020/formulas/chemistry/college/87jcayxsmac2e1rqssh252a15g1z4z468p.png) .
.
To form
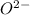 ion, this element will gain 2 electrons.
ion, this element will gain 2 electrons.
By criss-cross method, the oxidation state of the ions gets exchanged and they form the subscripts of the other ions. This results in the formation of a neutral compound.
So, the chemical formula for strontium oxide is
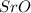
- For c: Aluminium and chlorine
Aluminium is the 13th element of periodic table having electronic configuration of
![[Ne]3s^23p^1](https://img.qammunity.org/2020/formulas/chemistry/middle-school/uud3u094nik5stg8236eujfz16gt2mxb3w.png)
To form
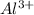 ion, this element will loose 3 electrons.
ion, this element will loose 3 electrons.
Chlorine is the 17th element of the periodic table having electronic configuration of
![[Ne]3s^23p^5](https://img.qammunity.org/2020/formulas/chemistry/high-school/e963fj2108sjpbrkolztmtjln5am3lxli2.png)
To form
 ion, this element will gain 1 electron.
ion, this element will gain 1 electron.
By criss-cross method, the oxidation state of the ions gets exchanged and they form the subscripts of the other ions. This results in the formation of a neutral compound.
So, the chemical formula for aluminium chloride is