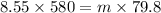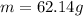Answer:
m = 62.14 g
Step-by-step explanation:
Energy used to melt the ice is the energy released by the condensation of the water forms on the glass
so here we have
energy for the condensation of water is given as
let mass of water condensed = m
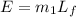
now the energy of vaporization is given as
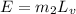
here we know that
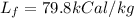
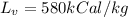
Now we have