Step-by-step explanation:
Number of oxygen molecules = 10 billion =
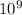
Number of hydrogen molecules = 10 billion =
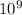
Number of molecules of hydrogen peroxides = 10 billion =
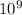
Moles of oxygen gas :
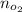
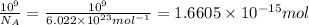
Moles of hydrogen gas :
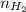
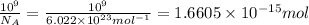
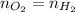
Moles of hydrogen gas :
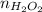
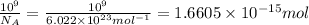
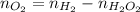
Number of moles in hydrogen gas, oxygen gas and hydrogen peroxide is same.
Total number of atoms in
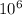 molecules of oxygen:
molecules of oxygen:
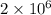 (2 atoms in 1 molecule)
(2 atoms in 1 molecule)
Total number of atoms in
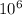 molecules of hydroegn :
molecules of hydroegn :
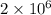 (2 atoms in 1 molecule)
(2 atoms in 1 molecule)
Total number of atoms in
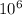 molecules of hydrogen peroxide:
molecules of hydrogen peroxide:
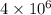 (4 atoms in 1 molecule)
(4 atoms in 1 molecule)
Number of oxygen atoms and number of hydrogen atoms are equal but individually they are not equal to number of atoms in hydrogen peroxide.