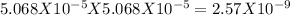Answer:
Ksp =
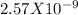
Step-by-step explanation:
Molar solubility of barium carbonate will be obtained by dividing the given solubility (in g/L) with molar mass of barium carbonate
molar solubility =
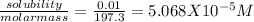
The barium carbonate will undergo dissociation as:

Ksp of barium carbonate will be
Ksp =
![[Ba^(+2)][CO_(3)^(-2)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ez8fly7w7guakmy02i9xs9pl8f4tws2jfl.png)
If solubility of barium carbonate is "s"
The concentration of each ion will be "s"
Hence the expression becomes
Ksp = s²
where
s = molar solubility = 5.068 X10⁻⁵
Ksp =