Answer:
The mass ratio of zinc to sulfide is 85:42.
2.5559 kg of Zn are in 3.82 kg of ZnS.
Step-by-step explanation:
a) Mass of zinc sulfide = 254 g
Mass of zinc in a zinc sulfide sample = 170 g
Mass of sulfide in zinc sulfide sample = x
254 g = 170 g+ x
x = 84 g
The mass ratio of zinc to sulfide:
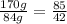
b) Mass of zincsulfide sample = 3.83 kg
The mass ratio of zinc to sulfide is 85:42.
Let the mass of zinc and sulfide be 85x and 42x respectively:
85 x+ 42 x=3.82 kg
x =0.03007 kg
Mass of an zinc= 85x=85 × 0.03007 kg= 2.5559 kg