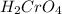Answer: The formula of chromic acid is
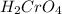
Step-by-step explanation:
We are given an ionic compound named as chromic acid. This acid is formed by the combination of hydrogen ion and chromate ion.
An acid is defined as the substance which releases hydrogen ion when dissolved in water.
Hydrogen is the 1st element of periodic table having electronic configuration of
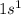 .
.
This element will loose 1 electron to form
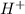 ion.
ion.
Chromate ion is a polyatomic ion having chemical formula of
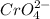
By criss-cross method, the oxidation state of the ions gets exchanged and they form the subscripts of the other ions. This results in the formation of a neutral compound.
So, the chemical formula for the given compound is
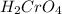
Thus, the formula of chromic acid is