Step-by-step explanation:
(a) Dry ice is solid carbon dioxide and its chemical formula is solid
 .
.
(b) Sodium chloride is commonly known as salt in daily life. Hence, chemical formula of salt is NaCl.
(c) Laughing gas is also known as dinitrogen monoxide. Hence, its chemical formula is
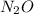 .
.
(d) Marble (chalk limestone) is chemically known as calcium carbonate. Hence, its chemical formula is
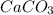 .
.
(e) Baking soda is chemically known as sodium bicarbonate. Hence, its chemical formula is
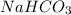 .
.
(f) Ammonia has a chemical formula as
 .
.
(g) Water has a chemical formula as
 .
.
(h) Milk of magnesia is a base and it is chemically known as magnesium hydroxide. Its chemical formula is
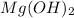 .
.
(i) Epsom salt is most often found as heptahydrate of sulfate mineral epsomite. Its chemical formula is usually
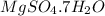 . If not present as a heptahydrate then its chemical formula is
. If not present as a heptahydrate then its chemical formula is
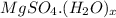 where, value of x can be equal to 0 to 7.
where, value of x can be equal to 0 to 7.