Answer:
Answer is given below.
Step-by-step explanation:
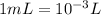
So,
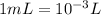 seawater contains
seawater contains
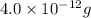 of gold
of gold
hence
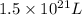 seawater contains
seawater contains
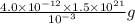 gold or
gold or
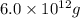 gold
gold
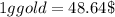
So,
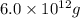 gold =
gold =
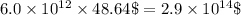
Seawater contains majorly different kinds of salts e.g.
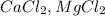 along with gold in very very small amount.
along with gold in very very small amount.
So, purification of seawater is more rigorous and more costlier than extracting gold from ores.
Hence investing money in mining gold from seawater lead to huge loss.