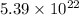Answer : The number of iron atoms present in 4.9 grams of iron are,
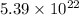
Explanation : Given,
As we are given that 1.0 grams of iron contains
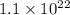 iron atoms.
iron atoms.
Now we have to determine the number of iron atoms in 4.9 grams.
As, 1.0 gram of iron contains
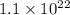 iron atoms
iron atoms
So, 4.9 gram of iron contains
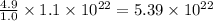 iron atoms
iron atoms
Therefore, the number of iron atoms present in 4.9 grams of iron are,