Answer:
Mass of sample is 99.9 g, density of ample is 11.35
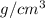 and temperature of sample is
and temperature of sample is
Step-by-step explanation:
Mass is an additive property. Therefore mass of combined sample is summation of masses of two pellets.
Mass of combined sample = (37.2+62.7) g = 99.9 g
Density is an intensive property. Therefore density of combined sample of lead will be same as with density of Pb.
Density of combined sample = 11.35
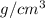
Temperature is an intensive property. Therefore temperature of combined sample of lead will be same as with individual pellets.
temperature of combined sample =