Answer:
\rho =2.70g/cm^3
Step-by-step explanation:
Hello,
Since density is defined as the degree of compactness of an object, it is mathematically defined via:
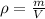
Thus, for the given mass and volume, one computes the density of aluminium as follows:
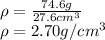
Best regards.