Answer:
 is the value of the equilibrium constant for this reaction.
is the value of the equilibrium constant for this reaction.
Step-by-step explanation:
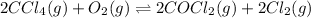
Molar concentrations of
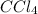 at equilibrium =
at equilibrium =
![[CCl_4]=1.0M](https://img.qammunity.org/2020/formulas/chemistry/college/9qstq2v3w33zblkmrhq4xopk1gpsn4bl6o.png)
Molar concentrations of
 at equilibrium =
at equilibrium =
![[O_2]=0.3M](https://img.qammunity.org/2020/formulas/chemistry/college/4lwxqpfi3veg6do3qbif9gm0524r1yifqb.png)
Molar concentrations of
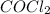 at equilibrium =
at equilibrium =
![[COCl_2]=4.0M](https://img.qammunity.org/2020/formulas/chemistry/college/jhx8wlnekaonbrx23dfkluz8kgiyghy2zm.png)
Molar concentrations of
 at equilibrium =
at equilibrium =
![[Cl_2]=2.0M](https://img.qammunity.org/2020/formulas/chemistry/college/di8o3lhk1pbavzyh82xigawhp2ncmq62vl.png)
The equilibrium constant is given as:
![K_c=([COCl_2]^2[Cl_2]^2)/([CCl_4]^2[O_2])=((4.0 M)^2* (2.0M)^2)/((1.0M)^2* (0.3 M))](https://img.qammunity.org/2020/formulas/chemistry/college/w217mzhe0cg8t20bio33arte0znffg0p6r.png)
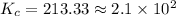
 is the value of the equilibrium constant for this reaction.
is the value of the equilibrium constant for this reaction.