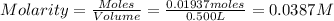Answer:
Ce concentration = 0.0387 M
Step-by-step explanation:
The given compound is: (NH₄)₂Ce(NO₃)₆
Mass = 10.6192 g
Mol. wt = 548.23 g/mol
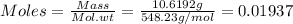
Based on the formula stoichiometry:
1 mole of (NH₄)₂Ce(NO₃)₆ contains 1 mole of Ce
Therefore, there are 0.01937 moles of Ce in the given compound
Volume of the solution = 500.0 ml = 0.500 L