Answer:
The heat is 10458 J and 15480 J.
Step-by-step explanation:
Given that,
Temperature T₁ = 404 K
Temperature T₂ = 298 K
Work done = 2740 J
If the temperature T₁ =298 k
Temperature T₂ = 245 K
We need to calculate the heat
Using efficiency formula
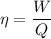 ...(I)
...(I)
We need to calculate the efficiency
Using formula of efficiency
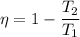
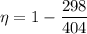
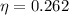
Put the value of efficiency in equation (I)
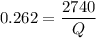
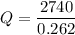
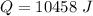
Again, we need to calculate the efficiency
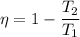
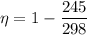
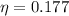
We need to calculate the heat
Using equation (I)
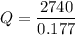
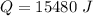
Hence, The heat is 10458 J and 15480 J.