Answer:
a)0.4222 moles of the compound are present.
b)
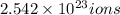 of iron(III)
of iron(III)
c)
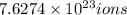 of bromide.
of bromide.
Step-by-step explanation:
Moles =
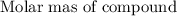
a)Moles of iron (III) bromide =
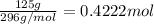
0.4222 moles of the compound are present.
Molecules of of iron (III) bromide in 0.4222 moles
1 mole =
 molecules
molecules
In , 0.4222 moles:
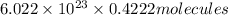
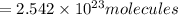 of iron (III) bromide
of iron (III) bromide
b)1 molecule of iron (III) bromide contain 1 iron(III) ion.Then
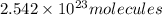 will contain:
will contain:
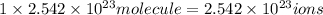 of iron(III)
of iron(III)
c) 1 molecule of iron (III) bromide contain 3 bromide ion.Then
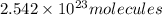 will contain:
will contain:
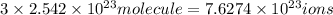 of bromide.
of bromide.