Answer: alpha particle
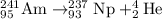
Step-by-step explanation:
Radioactive decay process is a type of process in which a less stable nuclei decomposes to a stable nuclei by releasing some radiations or particles like alpha, beta particles or gamma-radiations.
In a nuclear reaction, the total mass and total atomic number remains the same.
Alpha decay : When a larger nuclei decays into smaller nuclei by releasing alpha particle. In this process, the mass number and atomic number is reduced by 4 and 2 units respectively.
General representation of an element is given as:
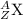
where,
Z represents Atomic number
A represents Mass number
X represents the symbol of an element
General representation of alpha decay :
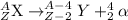
For the given reaction:
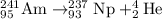
Thus a alpha particle is emitted on decay of a tiny amount of americium-241 to neptunium-237.