Answer:
Molar mass of ascorbic acid=176.13 g/mol
Number of moles in a ascorbic acids tablet contain 546 mg =
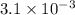
Number of molecules in a tablet of ascorbic acid of mass 546 mg=
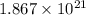 molecules
molecules
Step-by-step explanation:
We are given that ascorbic acid or vitamin C is an essential vitamin
We are given that given mass of ascorbic acid in a tablet =546.0 mg=
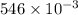
We have to find the number of moles and molecules of vitamin in C
We know that the chemical formula of ascorbic acid
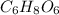
Molar mass of vitamin C =6(12.011)+8(1.0079)+6(16)=176.13 g/mol
Number of moles=
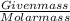
Number of moles of vitamin C=
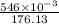
Number of moles of vitamin C=0.0031 moles=
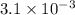
Number of molecules of vitamin C=
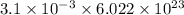
Where Avogadro's number =

Number of molecules of vitamin C=
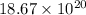
=
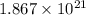 molecules
molecules