Answer:
Q = 41800[J]
Step-by-step explanation:
These types of problems can be solved using the following heat transfer equation for quarps that increase or decrease their temperature as a function of mass.
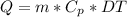
where:
Q = Heat absorbed [J]
m = mass = 2 [kg]
DT = temperature change = 5 [°C]
Cp = specific heat of the water = 4180 [J/kg*°C]
![Q=2*4180*5\\Q=41800[J]](https://img.qammunity.org/2022/formulas/physics/high-school/4f8y4fkpbmw4iynf5eeokoznlmvq02gufq.png)
Units of energy (Joules) [J]