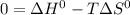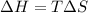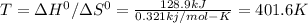Answer:
T = 401.6 K
Step-by-step explanation:
Given reaction:
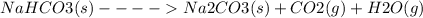
The thermodynamic parameters; ΔG°, ΔH° and ΔS° are related via the Gibbs -Helmholtz equation given as:
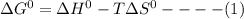
For a given reaction, the gibbs free energy change ΔG° is related to the equilibrium constant K as:
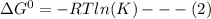
It is given that K = 1,
Therefore,
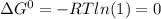
Substituting for ΔG° in equation (1)