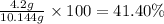Answer:
Percentage yield of 1,4-di-t-butyl-2,5-dimethoxybenzene is 41.40%.
Step-by-step explanation:
Here, in the reaction sulfuric acid is playing the role of catalyst by donating its proton in initial stage of the reaction and in the end of the reaction the proton is returned back to sulfuric acid.
Mass = Density × Volume
Mass of t-butyl alcohol =
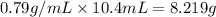
Moles of t-butyl alcohol =
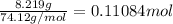
Moles of 1,4-dimethoxybenzene =
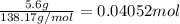
According to reaction 2 mol of t-butyl alcohol reacts with 1 mol of 1,4-dimethoxybenzene.
Then 0.11084 moles of t-butyl alcohol will react with :
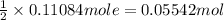 of 1,4-dimethoxybenzene.
of 1,4-dimethoxybenzene.
This means that moles of 1,4-dimethoxybenzene are limited and moles of t-butyl alcohol are in excess.So, the moles of product will depend upon the moles of 1,4-dimethoxybenzene.
According top reaction 1 mol of 1,4-dimethoxybenzene gives 1 mol of 1,4-di-t-butyl-2,5-dimethoxybenzene.
Then 0.04052 moles of 1,4-di-t-butyl-2,5-dimethoxybenzene will give:
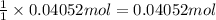 of 1,4-di-t-butyl-2,5-dimethoxybenzene.
of 1,4-di-t-butyl-2,5-dimethoxybenzene.
Mass of 0.04052 moles of 1,4-di-t-butyl-2,5-dimethoxybenzene:
0.04052 mol × 250.37 g/mol = 10.144 g
Percentage yield:
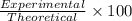
Percentage yield of 1,4-di-t-butyl-2,5-dimethoxybenzene:
Experimental yield = 4.2 g
Theoretical yield = 10.144 g