Answer:
Molarity of the base solution was 0.1830 M
Step-by-step explanation:
Total volume of NaOH added for complete standardization = (final reading-initial reading) = (30.89-1.98) mL = 28.91 mL
Neutralization reaction between NaOH and HCl :

So, 1 mol of HCl neutralizes 1 mol of NaOH
Moles of HCl added =
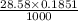 moles
moles
If molarity of NaOH was C (M) then moles of NaOH added is
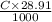
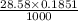 =
=
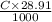
or, C = 0.1830
So, molarity of NaOH was 0.1830 M