Answer:
0.00415F
Step-by-step explanation:
Given parameters:
Current in the solution = 2A
Time = 200s
Volume of chlorine gas = 59.6mL
Pressure = 650mmHg
Temperature = 27° C
Unknown parameter:
The faraday which is the quantity of electricity used = ?
Solution:
The quantity of electricity used in the reaction is derived from the product of the current passed through the solution and time duration. It is expressed in coulombs.
Q = I x t
where I is the current
t is the time
Q = 2 x 200 = 400coulombs
One faraday is the quantity of electricity produced by one mole of electrons:
1Faraday = 96500 coulombs
Therefore 400coulombs gives
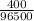 = 0.00415F
= 0.00415F