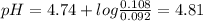Answer:
pH of buffer is 4.81
Step-by-step explanation:
The reaction between HCl and sodium acetate will product acetic acid. The presence of weak acid (acetic acid) and its salt will make a buffer.
The reaction will be:

Thus one mole each of HCl and acetate will give one mole of acetic acid.
The moles of HCl present = molarity X volume = 0.452 X 0.204 =0.092
moles of acetic acid formed on reaction of HCl with acetate = 0.092
Initial moles of sodium acetate present = molarity X volume = 0.4X0.5 =0.2
moles of sodium acetate left =0.108 mol
the pKa of acetic acid =4.74
The pH of buffer is calculated using Henderson Hassalbalch's equation :
pH = pKa + log
![([salt])/([acid])](https://img.qammunity.org/2020/formulas/chemistry/college/vzxm9t2bqf38z1vxhn60zy3sdrgifjnpl8.png)