Answer:
molecular formula = C₁₀H₂₀O
Step-by-step explanation:
the carbon dioxide is obtained on combustion from an organic molecule due to reaction of carbon with oxygen and water is formed due to reaction of hydrogen with oxygen to form water.
The reactions are:
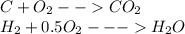
From above equations
i) 44g of carbon dioxide is formed from 12g of carbon
Therefore 0.2829g of carbon dioxide must be formed from =
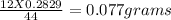 of carbon
of carbon
ii) 18g of water is formed from 2 grams of hydrogen
Therefore 0.1159 grams of water must be formed from =
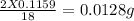 hydrogen
hydrogen
Total weight of sample taken = 0.1005
so mass of oxygen = 0.1005-(0.077+0.0129)=0.0106 g
moles of carbon = mass / atomic mass = 0.077/12=0.00641
moles of hydrogen = mass /atomic mass = 0.0128/1=0.0128
moles of oxygen = mass / atomic mass =0.0106/ 16=0.00066
Let us divide all the moles with the smallest one
moles of carbon = 0.00641 / 0.00066 = 10 (approx)
moles of hydrogen = 0.0128/0.00066 = 20 (approx)
moles of oxygen = 0.00066/0.00066 = 1
Empirical formula of menthol = C₁₀H₂₀O
Empirical mass =10X12 + 20X1 + 16= 156
Which is equal to molar mass hence the empirical formula = molecular formula