Answer:
Final temperature: 659.8ºC
Expansion work: 3*75=225 kJ
Internal energy change: 275 kJ
Step-by-step explanation:
First, considering both initial and final states, write the energy balance:
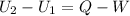
Q is the only variable known. To determine the work, it is possible to consider the reversible process; the work done on a expansion reversible process may be calculated as:
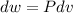
The pressure is constant, so:
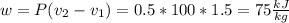 (There is a multiplication by 100 due to the conversion of bar to kPa)
(There is a multiplication by 100 due to the conversion of bar to kPa)
So, the internal energy change may be calculated from the energy balance (don't forget to multiply by the mass):
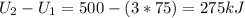
On the other hand, due to the low pressure the ideal gas law may be appropriate. The ideal gas law is written for both states:
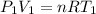
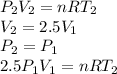
Subtracting the first from the second:
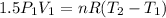
Isolating
 :
:
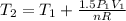
Assuming that it is water steam, n=0.1666 kmol
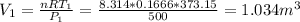
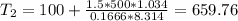 ºC
ºC