Answer:15 KJ
Step-by-step explanation:
We have given
at 1 atm
The heat of sublimation of Gallium =277 KJ/mol
heat of vaporization of Gallium =271 KJ/mol
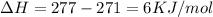
So heat required to melt 2.50 mol of gallium at 1 atm
Heat Required=
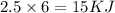
So heat required to melt 2.5 mol of gallium is 15 KJ