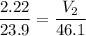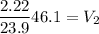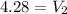Answer:
Volume = 4.28L
Step-by-step explanation:
Charles's gas law relates the volume and temperature of a gas at constant pressure. This law says that at constant pressures if we raise the temperature of a gas it will expand and if we reduce the temperature the volume will decrease. The formula is as follows:
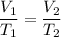
So, the initial conditions are 2.22L and 23.9 °C ant the final conditions are 46.1 °C we replace them in the equation. And then we solve it.