Answer : The number of moles of gas added to the container will be, 20.89 mole
Explanation :
According to the Avogadro's law, the volume of gas is directly proportional to the number of moles of gas at same pressure and temperature. That means,
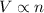
or,
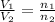
where,
 = initial volume of gas = 8.15 L
= initial volume of gas = 8.15 L
 = final volume of gas = 17.9 L
= final volume of gas = 17.9 L
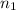 = initial number of moles of gas = 9.51 mole
= initial number of moles of gas = 9.51 mole
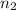 = final number of moles of gas = ?
= final number of moles of gas = ?
Now we put all the given values in this formula, we get the final moles of gas.


Therefore, the number of moles of gas added to the container will be, 20.89 mole