Answer:
127.28 mmHg
Step-by-step explanation:
The molar fraction of oxygen in dry air at 760 mmHg is 20.9%. This molar fraction is not affected too much by the height, so it may be taken as a constant. The partial pressure of oxygen may be calculated as:
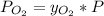
So, if the total pressure is 609 mmHg,
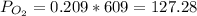 mmHg.
mmHg.