Answer:
The nuclear binding energy =
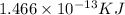
The binding energy per nucleon =1.37×10⁻¹⁵ KJ/nucleon
Step-by-step explanation:
Given:
Number of protons = 47
Number of neutrons = 107-47 = 60
Now,
the mass defect (m)= Theoretical mass - actual mass
m =
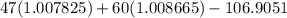
since,
mass of proton = 1.007825 amu
Mass of neutron = 1.008665 amu
thus,
m =
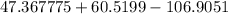
or
m =
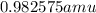
also
1 amu =
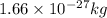
therefore,
m =
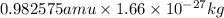
or
m =
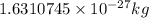
now,
Energy = mass × (speed of light)²
thus,
Energy =
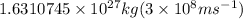
or
Energy =
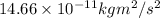
or
Energy =
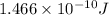 =
=
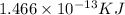
Therefore the nuclear binding energy =
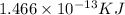
Now,
the binding energy per nucleon =
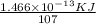 = 1.37×10⁻¹⁵ KJ/nucleon
= 1.37×10⁻¹⁵ KJ/nucleon