Answer:
The concentration of NaCl = 0.3374 M
Step-by-step explanation:
Given :
Molarity of AgNO₃ = 0.2503 M
Volume of AgNO₃ = 20.22 mL
The conversion of mL into L is shown below:
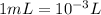
Thus, volume of the solution = 20.22×10⁻³ L
Molarity of a solution is the number of moles of solute present in 1 L of the solution.

The formula can be written for the calculation of moles as:

Thus,
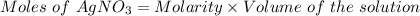
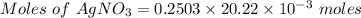
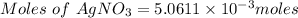
The chemical reaction taking place:
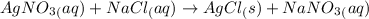
According to reaction stoichiometry:
1 mole of AgNO₃ reacts with 1 mole of NaCl
Thus,
5.0611×10⁻³ moles of AgNO₃ reacts with 5.0611×10⁻³ moles of NaCl
Thus, moles of NaCl required = 5.0611×10⁻³ moles
Volume of NaCl required = 15.00 mL
The conversion of mL into L is shown below:
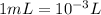
Thus, volume of the solution = 15.00×10⁻³ L
Applying in the formula of molarity as:

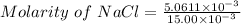
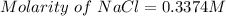
Thus, the concentration of NaCl = 0.3374 M