Answer:
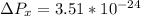 kg m/s
kg m/s
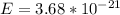 J
J
Step-by-step explanation:
given data
uncertainty in proton position
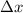 = 0.015 nm
= 0.015 nm
according to Heisenberg's principle of uncertainty
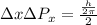
Where h is plank constant =
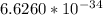 j-s
j-s
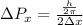
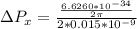
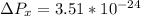 kg m/s
kg m/s
b) kinetic energy of proton whose momentum
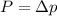
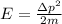
where m is mass is proton
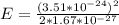
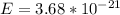 J
J