Answer: The mass of sulfur hexafluoride is 146 u.
Step-by-step explanation:
Mass of a mole of a substance is defined as the molar mass of that substance.
It is defined as the sum of every component each multiplied by the times they appear in that substance.
We are given:
Mass of sulfur atom = 32.0 u
Mass of fluorine atom = 19.0 u
The chemical formula for sulfur hexafluoride is
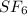
So, the molar mass of
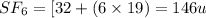
Hence, the mass of sulfur hexafluoride is 146 u.